Advancements in Automated Colony Phenotyping for High-Throughput Microbiology
1. Introduction: The Enduring Value of Solid Culture
Solid culture on agar plates has been the foundational technique of microbiology since the work of Robert Koch, providing the most reliable method for isolating single bacterial clones and quantifying microbial populations in terms of Colony Forming Units (CFUs). While liquid culture assays offer speed for population-averaged metrics (e.g., OD600), solid culture provides the crucial advantage of spatial separation, which is a prerequisite for observing the unique phenotypic characteristics of individual, clonal populations.
The primary limitation of this legacy technique has been its low throughput, relying on slow, manual observation and counting. This bottleneck has become untenable in modern fields like Synthetic Biology and Strain Engineering, where millions of genetic variants must be screened and assessed for subtle differences in fitness and productivity. The development of automated systems for measuring bacterial growth on solid culture represents a critical advancement, transforming the legacy technique into a powerful, high-throughput tool for quantitative phenotyping.
2. The Quantitative Limitations of Traditional CFU Assays
The traditional approach of plating, incubation, and manual counting captures only an endpoint measurement. This CFU count determines the total number of viable cells but provides no kinetic data about how those cells grew, adapted, or formed colonies over time. This lack of kinetic insight, coupled with logistical drawbacks, severely limits its utility for modern strain optimization:
- Endpoint-Only Measurement: Without time-resolved data, researchers cannot measure crucial kinetic parameters like the lag phase or the maximum exponential growth rate of individual clones. These parameters are critical indicators of strain robustness and metabolic burden [6].
- Subjective Phenotyping: Characteristics like colony size, texture, and color are typically noted qualitatively, preventing the precise, quantitative comparison needed to differentiate high-performing engineered variants from wild-type populations.
- Counting Errors at High Density: In the high-density plating required for large-scale screening, colonies frequently merge. Manual and older automated systems often count these merged areas as single units, leading to significant underestimation of the true CFU count [5].
These limitations ensure that traditional solid culture remains inefficient for the Design-Build-Test-Learn (DBTL) cycle, where continuous, quantitative feedback is necessary for rapid genetic optimization.
3. Advancements in Automated Solid Culture Growth Monitoring
The evolution of automated systems integrates specialized hardware (incubator-enclosed scanners or cameras) with advanced computer vision to provide non-invasive, high-resolution monitoring. These platforms enable true high-throughput colony phenotyping by tracking the kinetics of thousands of individual clones simultaneously.
3.1. Time-Lapse Systems: ScanLag and ColTapp
Early generations of automated monitoring replaced the manual endpoint count with time-lapse kinetic analysis of colony growth, providing every colony with a quantitative signature:
- The ScanLag Methodology: Pioneer systems like ScanLag demonstrated the power of non-invasive, periodic image acquisition to track colony growth curves. By measuring the area of every clone over time, ScanLag provided the first practical method for characterizing a two-dimensional distribution of both the adaptation time (lag time) and the colony growth rate for millions of cells [1].
- ColTapp for Growth Quantification: Dedicated software such as ColTapp (Colony Time-lapse application) refined this analysis, focusing on robust image processing to accurately quantify colony growth and lag time dynamics. This is crucial for applications like studying antibiotic persistence, where subtle differences of the lag time are critical [2].
3.2. Next-Generation Monitoring: Pre-Colony Detection
The most advanced growth monitoring systems use high-resolution sensor arrays to detect transmitted light rather than reflected light, pushing the detection limit beyond the emergence of a macroscopic colony.
- Micro-Colony Optical Density: The next-generation Bacterial Growth Monitor (BGM), developed by Carbgem, utilizes large (10 cm x 10 cm) two-dimensional sensors that capture data at a high spatial resolution of 1,000 x 1,000 pixels without a lens, as well as at a high temporal frequency of every 5 minutes. The system performs optical density (OD) measurements directly on the solid medium [3].
- Detection Before Visible Colony: By measuring the subtle changes in light scattering caused by a cluster of just a few cells (a micro-colony), the system can detect the onset of growth even before a physical colony has visibly formed [3]. This capability can shave hours off the assay time—a crucial advantage when screening for rapidly acting inhibitory compounds or extremely slow-growing engineered strains.
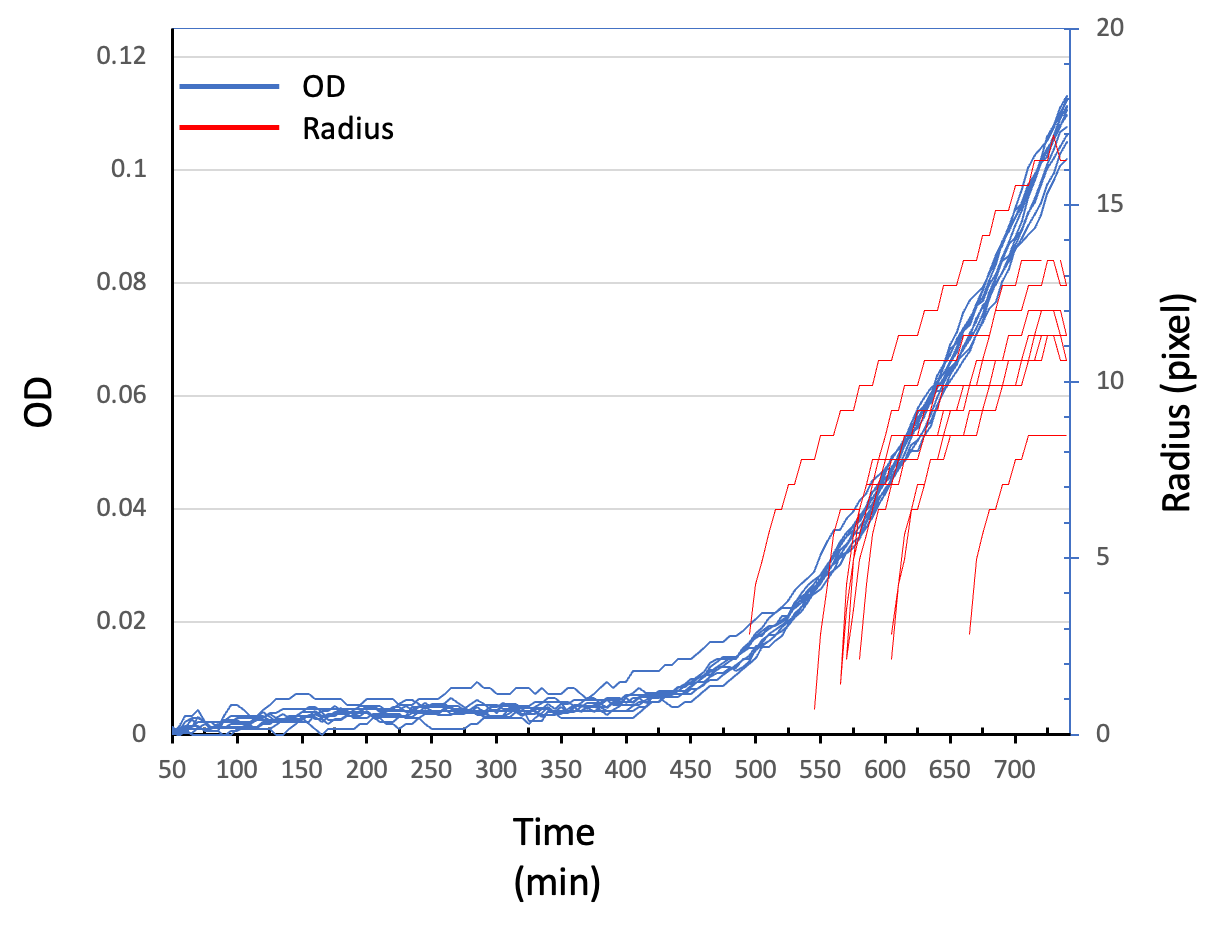
In this experiment, five BGMs and the camera apparatus were set in the same incubator (Figure 1a). Image analysis at earlier time points showed that BGM detected the increase of OD at 400-450 min, whereas the camera detected the increase of the radial growth at 500-550 min (Figure 1b).
Figure 1 (a) BGM (left) and the camera apparatus (right) in the same incubator. (b) The OD curves of the 10 fastest growing pixels detected by BGM (blue) and the radius curves of the first 10 colonies detected from the camera images.
- Enhanced Phenomics: The data stream—high-resolution kinetic and OD data for thousands of individual sites—significantly enriches the dataset for machine learning models, leading to more accurate predictions of inhibition, toxicity, and ultimate strain performance [3].
3.3. Hardware Integration and High-Throughput Retrieval
The integration of advanced kinetics with robotics transforms the screening process:
- Merged Colony Resolution: Sophisticated image processing algorithms (e.g., MCount) are essential to accurately deconvolve overlapping colonies, correcting the severe counting underestimation in high-density plating [5].
- Data-Driven Selection: The quantitative metrics of growth monitors (lag time, growth rate, pre-colony OD curve) are integrated with robotic colony pickers [4]. This enables the objective selection of clones based on a specific, multi-parameter profile—for instance, selecting clones that show a quick lag time but then grow slowly (indicating high metabolic burden/productivity), ensuring the best possible variant is retrieved for scale-up [6].
4. Impact on Strain Screening and Clinical Microbiology
The shift to quantitative solid-culture monitoring overcomes a fundamental bottleneck in the bio-engineering pipeline.
4.1. Decoupling Fitness and Production
The greatest advantage in strain engineering is solving the “rare variant bottleneck.” Since engineered strains often suffer a metabolic burden leading to slower growth, they are quickly lost in liquid culture due to competition [7]. On solid culture, the spatial separation and kinetic monitoring ensure:
- Protection from Competition: Each engineered cell is safe within its own colony, allowing the slow-growing, highly productive variant to survive and be detected.
- Decoupled Phenotypes: The system measures the colony’s growth dynamics (fitness) and its phenotypic trait (e.g., product-correlated color or micro-colony OD) separately, allowing researchers to select for the optimal balance of both characteristics.
4.2. Applications in Clinical and Discovery Research
The detailed kinetic data provided by these systems are highly relevant across research fields:
- Antibiotic Discovery: The ability to detect zones of inhibition in disk diffusion test and growth curves with hours of greater lead time significantly accelerates the search for new antibiotics, peptides, and bacteriophages [3, 9].
- Bacterial Persistence: Precise measurement of the adaptation time for individual clones is used to study the drug-tolerant “persister” mechanism, crucial for understanding chronic and recurrent infections [2, 9].
5. Conclusion
Automated solid-culture monitoring systems, evolving from time-lapse methodologies like ScanLag and ColTapp to the advanced Bacterial Growth Monitor, have successfully digitized and quantified the legacy CFU assay. By providing high-throughput, time-resolved kinetic data on individual clones, these platforms eliminate the subjectivity and limitations of manual screening. They offer the necessary resolution to overcome the critical bottleneck in both synthetic biology and industrial microbiology, ensuring that the rare, elite variants—the keys to successful biomanufacturing and advanced diagnostics—are reliably identified and retrieved.
6. References
- Levin-Reisman, I., et al. (2014). ScanLag: High-throughput Quantification of Colony Growth and Lag Time. Journal of Visualized Experiments, (89):51456. link
- Bar, J., et al. (2020). Efficient microbial colony growth dynamics quantification with ColTapp, an automated image analysis application. Scientific Reports, 10, 16084. link
- Taketani, M., et al. (2023). Image Sensor-Based Real Time Monitoring of Bacterial Growth on Agar Plates. IDWeek, Boston.
- Molecular Devices. (2025). Clone Screening Solutions, Automated Colony Picking. link
- Chen, S., et al. (2025). MCount: An automated colony counting tool for high-throughput microbiology. PLoS One, 20 (3), e0311242. link
- Abbate, E., et al. (2023). Optimizing the strain engineering process for industrial-scale production of bio-based molecules. Journal of Industrial Microbiology and Biotechnology, 50(1), kuad025. link
- Jiang, Y., et al. (2023). Strain and process engineering toward continuous industrial fermentation. Frontiers of Chemical Science and Engineering, 17(6), 632–641. link
- Jin, C., et al. (2022). High-throughput identification and quantification of single bacterial cells in the microbiota. Nature Communications, 13(1), 896. link
- CarbGeM Inc. (2025). Bacterial Growth Monitor (BGM). link